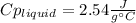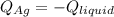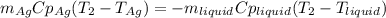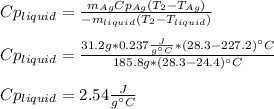Chemistry, 16.07.2020 01:01 makaylaf9479
3. A 31.2-g piece of silver (s = 0.237 J/(g · °C)), initially at 277.2°C, is added to 185.8 g of a liquid, initially at 24.4°C, in an insulated container. The final temperature of the metal–liquid mixture at equilibrium is 28.3°C. What is the specific heat of the liquid? Neglect the heat capacity of the container.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3

You know the right answer?
3. A 31.2-g piece of silver (s = 0.237 J/(g · °C)), initially at 277.2°C, is added to 185.8 g of a l...
Questions

Mathematics, 22.12.2020 01:20

Medicine, 22.12.2020 01:20

Mathematics, 22.12.2020 01:20



Mathematics, 22.12.2020 01:20

Mathematics, 22.12.2020 01:20




Computers and Technology, 22.12.2020 01:20





Chemistry, 22.12.2020 01:20


Spanish, 22.12.2020 01:20