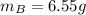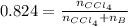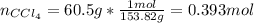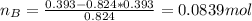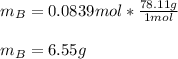Chemistry, 16.07.2020 01:01 jennamcasey94
A solution was made by mixing benzene () and carbon tetrachloride (). Given that the mole fraction of carbon tetrachloride is 0.824 in the solution obtained from 60.5 g , calculate the mass of benzene used. Mass

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5 m hcl? show all of the work needed to solve this problem. mg (s) + 2hcl (aq) → mgcl2 (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1
You know the right answer?
A solution was made by mixing benzene () and carbon tetrachloride (). Given that the mole fraction o...
Questions



Mathematics, 03.03.2021 08:30

Mathematics, 03.03.2021 08:30


History, 03.03.2021 08:30

Mathematics, 03.03.2021 08:30

Mathematics, 03.03.2021 08:30

Mathematics, 03.03.2021 08:30

Social Studies, 03.03.2021 08:30


English, 03.03.2021 08:30

Biology, 03.03.2021 08:30

Mathematics, 03.03.2021 08:30

Mathematics, 03.03.2021 08:30




Mathematics, 03.03.2021 08:30

English, 03.03.2021 08:30