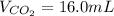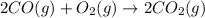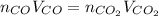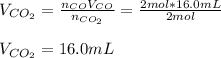Chemistry, 15.07.2020 09:01 westlakebuddy1229
Assuming the same temperature and pressure for each gas, how many milliliters of carbon dioxide are produced from 16 0 mL of CO
2 CO(g) + O2(g) 4, 2 CO2 (g)
Express your answer with the appropriate units.

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

You know the right answer?
Assuming the same temperature and pressure for each gas, how many milliliters of carbon dioxide are...
Questions


English, 07.12.2020 18:50

Arts, 07.12.2020 18:50

Mathematics, 07.12.2020 18:50

Biology, 07.12.2020 18:50

Mathematics, 07.12.2020 18:50

Mathematics, 07.12.2020 18:50


English, 07.12.2020 18:50

Mathematics, 07.12.2020 18:50

Mathematics, 07.12.2020 18:50




Social Studies, 07.12.2020 18:50


English, 07.12.2020 18:50

Mathematics, 07.12.2020 18:50