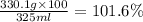Chemistry, 15.07.2020 05:01 pgjohnston001
Calculate the mass percent by volume of 330.1 g of glucose (C₆H₁₂O₆, MM = 180.2 g/mol) in 325 mL of solution.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
If the same amount of cacl2 is added to equal volumes of water and maple syrup, which will have the higher temperature?
Answers: 1

Chemistry, 22.06.2019 01:10
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 06:30
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1
You know the right answer?
Calculate the mass percent by volume of 330.1 g of glucose (C₆H₁₂O₆, MM = 180.2 g/mol) in 325 mL of...
Questions

Mathematics, 11.03.2021 01:30






Mathematics, 11.03.2021 01:30

Mathematics, 11.03.2021 01:30


Mathematics, 11.03.2021 01:30


Mathematics, 11.03.2021 01:30

Medicine, 11.03.2021 01:30

Mathematics, 11.03.2021 01:30



Mathematics, 11.03.2021 01:30


Mathematics, 11.03.2021 01:30

Chemistry, 11.03.2021 01:30