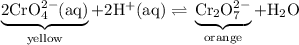Chemistry, 15.07.2020 05:01 nasrul3725
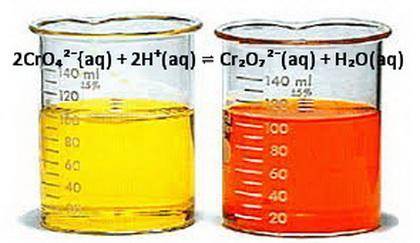
Note the dynamic equilibrium in the opening photo which solution changes color when the pH of both solutions is increased explain?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Problem #3 (ch. 1, problem 15)the ideal gas law provides one way to estimate the pressure exerted by a gas on a container. the law isí‘ťí‘ť=푛푛푛푛푛푛푉푉more accurate estimates can be made with the van der waals equationí‘ťí‘ť=푛푛푛푛푛푛푉푉â’푛푛푟푟â’푞푞푛푛2푉푉2where the term nb is a correction for the volume of the molecules and the term an2/v2is a correction for molecular attractions. the values of a and b depend on the type of gas. the gas constant is r, the absolutetemperature is t, the gas volume is v, and the number of moles of gas molecules is indicated by n. if n = 1 mol of an ideal gas were confined to a volume of v = 22.41 l at a temperature of 0â°c (273.2k), it would exert a pressure of 1 atm. in these units, r = 0.0826.for chlorine gas (cl2), a = 6.49 and b = 0.0562. compare the pressure estimates given by the ideal gas law and the van der waals equation for 1 mol of cl2 in 22.41 l at 273.2 k. what is the main cause of the difference in the two pressure estimates, the molecular volume or the molecular attractions?
Answers: 1

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 05:40
Salicylic acid is a very important acid. it is used to synthesize the aspirin by treating with acetic anhydride. a 0.2015-g sample of salicylic acid was dissolved in a 100.00-ml volumetric flask, and the solution was diluted to the mark. a 10-ml aliquot of this solution was titrated with standard naoh (0.01130 + 0.2% n) to a phenolphthalein faint pink color end point at 19.81 ml. (a) (calculate the normality of the salicylic acid solution used in the titration. (b) assuming the salicylic acid is pure, what is the equivalent weight of the salicylic acid? practice problems for the final exam (continued) (c) (calculate the inherent error in the determination of the equivalent weight you calculated in part (b). use the following absolute errors in the equipment /glassware when calculating the inherent error. 5.00-ml pipet: + 0.02 ml 100-ml volumetric flask: + 0.08 ml analytical balance: + 0.2 mg 25-ml buret: + 0.03 ml
Answers: 2

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
You know the right answer?
Note the dynamic equilibrium in the opening photo which solution changes color when the pH of both s...
Questions

Mathematics, 11.09.2020 09:01

Mathematics, 11.09.2020 09:01

Mathematics, 11.09.2020 09:01

Mathematics, 11.09.2020 09:01

Mathematics, 11.09.2020 09:01

Mathematics, 11.09.2020 09:01

Biology, 11.09.2020 09:01

Mathematics, 11.09.2020 09:01

Mathematics, 11.09.2020 09:01

Mathematics, 11.09.2020 09:01

Mathematics, 11.09.2020 09:01

Mathematics, 11.09.2020 09:01

Mathematics, 11.09.2020 09:01

Social Studies, 11.09.2020 09:01

Mathematics, 11.09.2020 09:01

Mathematics, 11.09.2020 09:01

Mathematics, 11.09.2020 09:01

Mathematics, 11.09.2020 09:01

Mathematics, 11.09.2020 09:01

Mathematics, 11.09.2020 09:01