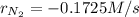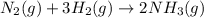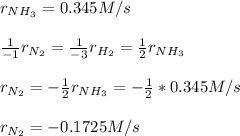Chemistry, 15.07.2020 04:01 jmathematics7806
In the Haber process, nitrogen gas is combined with hydrogen (from natural gas) to form ammonia. If ammonia is formed at 0.345 M/s, how quickly is the nitrogen gas disappearing

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:50
Your roll: experienced electron speech is adressed to: a new "freshman class" of electrons job: write a speech task: you are to pretend that you are giving a speech to a new group of electrons. be sure to mention their placement in an atom, their charge, and their role in chemical bonding (ionic and covalent) be specific!
Answers: 3

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

You know the right answer?
In the Haber process, nitrogen gas is combined with hydrogen (from natural gas) to form ammonia. If...
Questions

Social Studies, 28.02.2021 19:00

Mathematics, 28.02.2021 19:00

Social Studies, 28.02.2021 19:00

Mathematics, 28.02.2021 19:00


German, 28.02.2021 19:00

Social Studies, 28.02.2021 19:00

History, 28.02.2021 19:00

English, 28.02.2021 19:00

Arts, 28.02.2021 19:00

Mathematics, 28.02.2021 19:00


Mathematics, 28.02.2021 19:00

Mathematics, 28.02.2021 19:00



Geography, 28.02.2021 19:00

Social Studies, 28.02.2021 19:00


English, 28.02.2021 19:00