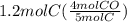Chemistry, 14.07.2020 03:01 xXCoryxKenshinXx
How many moles of CO are produced when 1.2 moles C reacts? Equation: 5C(s)+2SO2(g)→CS2(l)+4CO(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
You know the right answer?
How many moles of CO are produced when 1.2 moles C reacts? Equation: 5C(s)+2SO2(g)→CS2(l)+4CO(g)...
Questions




Mathematics, 15.05.2021 04:50

History, 15.05.2021 04:50



Mathematics, 15.05.2021 04:50

Advanced Placement (AP), 15.05.2021 04:50


Social Studies, 15.05.2021 04:50


Mathematics, 15.05.2021 04:50


Mathematics, 15.05.2021 04:50




Mathematics, 15.05.2021 04:50