
Chemistry, 14.07.2020 02:01 sandyrose3012
A solution was prepared by dissolving 195.0 g of KCl in 215 g of water. Calculate the mole fraction of KCl. (The formula weight of KCl is 74.6 g/mol. The formula weight of water is 18.0 g/mol.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 23.06.2019 06:30
Which of these describes how heat is transferred by convection* a. sunlight travels through space without the aid of fluids or solids. b. warm air rises and takes the heat with it, eventually, it cools and sinks c. air at the equator rises and sinks at the poles. d. air molecules touch the warm ground, heating them up *not conduction
Answers: 3

Chemistry, 23.06.2019 15:30
Amole, which is a unit in chemistry, contains 6.02 x 1023 atoms or particles. how many zeroes follow the 2 when this number is written out in standard form? a) 21 b) 23 c) 24 d) 25
Answers: 1

Chemistry, 23.06.2019 19:00
What is the final temperature after 840 joules is absorbed by 10.0g of water at 25.0c
Answers: 1
You know the right answer?
A solution was prepared by dissolving 195.0 g of KCl in 215 g of water. Calculate the mole fraction...
Questions

English, 26.10.2021 16:10






Biology, 26.10.2021 16:10



English, 26.10.2021 16:10

Mathematics, 26.10.2021 16:10


Mathematics, 26.10.2021 16:10




Mathematics, 26.10.2021 16:10

History, 26.10.2021 16:10

Mathematics, 26.10.2021 16:10

Law, 26.10.2021 16:10

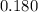 .
.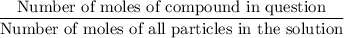 .
.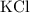 in this solution would be:
in this solution would be: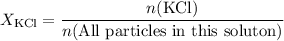 .
.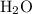 .) Hence:
.) Hence: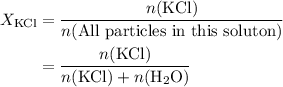 .
.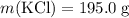 .Molar mass of
.Molar mass of 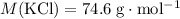 .Mass of
.Mass of 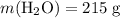 .Molar mass of
.Molar mass of  .
.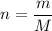 to find the number of moles of
to find the number of moles of  .
.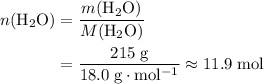 .
. .
.

