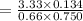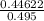When H2O and CO react at 979°C, the products are CO2 and H2. The equilibrium constant (in terms of equilibrium concentrations of reactants and products) for the reaction below is 0.66 at 979°C. If the following concentrations are measured after the reaction reaches equilibrium, what is the concentration of CO(g) in the equilibrated mixture? answer will be in M
Component: Measured Equilibrium Concentration
A. H2 0 (g) 0.750 M
B. CO2 (g) 0.134 M
C. H2 (g) 3.33 M

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2
You know the right answer?
When H2O and CO react at 979°C, the products are CO2 and H2. The equilibrium constant (in terms of e...
Questions


Mathematics, 03.02.2021 01:00






Mathematics, 03.02.2021 01:00




Health, 03.02.2021 01:00

Mathematics, 03.02.2021 01:00


Computers and Technology, 03.02.2021 01:00


Computers and Technology, 03.02.2021 01:00


Social Studies, 03.02.2021 01:00

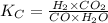
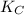 = equilibrium constant
= equilibrium constant