
Chemistry, 15.07.2020 01:01 Nathaliasmiles
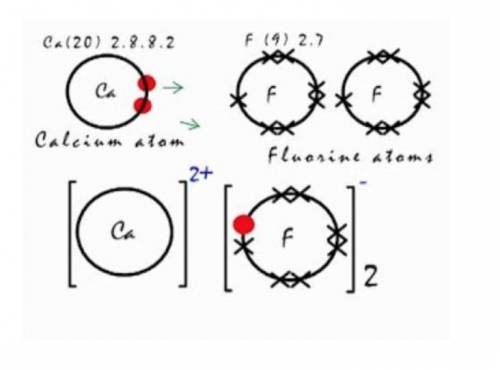
Q10. Calcium fluoride can be made from the reaction of calcium metal with fluorine gas. The image shows this reaction. Explain how the product is formed in terms of electron movement and what the final electron configurations are

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3
You know the right answer?
Q10. Calcium fluoride can be made from the reaction of calcium metal with fluorine gas. The image sh...
Questions

Mathematics, 02.03.2021 19:00

Chemistry, 02.03.2021 19:00

English, 02.03.2021 19:00

SAT, 02.03.2021 19:00


Biology, 02.03.2021 19:00

Mathematics, 02.03.2021 19:00



Chemistry, 02.03.2021 19:00



English, 02.03.2021 19:00




Mathematics, 02.03.2021 19:00



Mathematics, 02.03.2021 19:00



