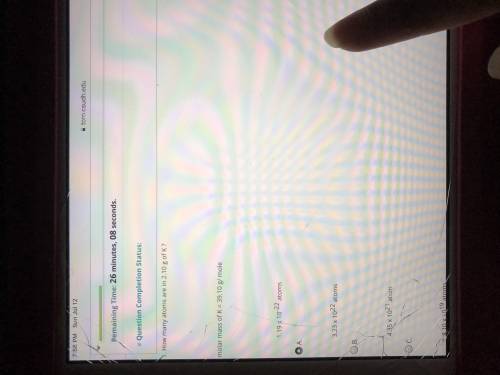
How many atoms are in 2.10 g of K? molar mass of K=39.10g/mole.
...


Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What were the success and failures that came to boyle’s excitements?
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
Questions

Chemistry, 06.10.2019 10:10





History, 06.10.2019 10:10

Social Studies, 06.10.2019 10:10


History, 06.10.2019 10:10


History, 06.10.2019 10:10


Business, 06.10.2019 10:10


Spanish, 06.10.2019 10:10



Mathematics, 06.10.2019 10:10


History, 06.10.2019 10:10