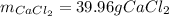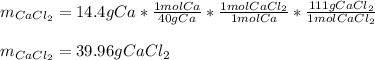Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1
You know the right answer?
For the reaction Ca(s)+Cl2(g)→CaCl2(s) calculate how many grams of the product form when 14.4 g of C...
Questions



Physics, 14.09.2021 19:20

Mathematics, 14.09.2021 19:20

Mathematics, 14.09.2021 19:20

Mathematics, 14.09.2021 19:20


Chemistry, 14.09.2021 19:20



Social Studies, 14.09.2021 19:20


Mathematics, 14.09.2021 19:20


Social Studies, 14.09.2021 19:20



Mathematics, 14.09.2021 19:20


Mathematics, 14.09.2021 19:20