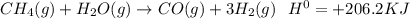Chemistry, 15.07.2020 01:01 gtemple22pdzs4j
The equilibrium reaction below has the Kc = 4.00 at 25°C. If the temperature of the system at equilibrium is increased to 100°C, how and for what reason will the equilibrium shift. Also show and explain how and why the Kc value will change.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3

You know the right answer?
The equilibrium reaction below has the Kc = 4.00 at 25°C. If the temperature of the system at equili...
Questions








Mathematics, 03.10.2019 00:30

Biology, 03.10.2019 00:30


Mathematics, 03.10.2019 00:30





Mathematics, 03.10.2019 00:30

English, 03.10.2019 00:30


History, 03.10.2019 00:30

Mathematics, 03.10.2019 00:30