
Chemistry, 14.07.2020 02:01 20emmanuelg1030
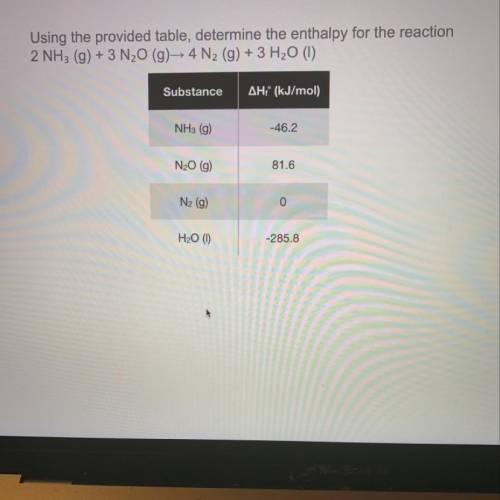
Using the provided table, determine the enthalpy for the reaction 2 NH3 (g) + 3 N20 (g) 4 N2 (g) + 3 H20 (1)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1
You know the right answer?
Using the provided table, determine the enthalpy for the reaction
2 NH3 (g) + 3 N20 (g) 4 N2 (g) +...
Questions


History, 14.04.2020 20:29


English, 14.04.2020 20:29

Mathematics, 14.04.2020 20:29

Biology, 14.04.2020 20:29




English, 14.04.2020 20:29


Mathematics, 14.04.2020 20:29


History, 14.04.2020 20:29

Geography, 14.04.2020 20:29





English, 14.04.2020 20:30



