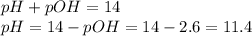Chemistry, 15.07.2020 01:01 29delphina
What is the pH of a 0.300 M NH₃ solution that has Kb = 1.8 × 10⁻⁵ ? The equation for the dissociation of NH₃ is: NH₃ (aq) + H₂O (l) ⇄ NH₄⁺ (aq) + OH⁻ (aq)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3
You know the right answer?
What is the pH of a 0.300 M NH₃ solution that has Kb = 1.8 × 10⁻⁵ ? The equation for the dissociatio...
Questions

Social Studies, 03.02.2021 21:50

Mathematics, 03.02.2021 21:50

Mathematics, 03.02.2021 21:50

Computers and Technology, 03.02.2021 21:50

French, 03.02.2021 21:50

Mathematics, 03.02.2021 21:50

Mathematics, 03.02.2021 21:50

History, 03.02.2021 21:50

Mathematics, 03.02.2021 21:50



History, 03.02.2021 21:50

History, 03.02.2021 21:50

Mathematics, 03.02.2021 21:50

History, 03.02.2021 21:50



Mathematics, 03.02.2021 21:50

Geography, 03.02.2021 21:50

![[OH^{-} ]=\sqrt{Kb \times Cb } = \sqrt{1.8 \times 10^{-5} \times 0.300 } = 2.3 \times 10^{-3} M](/tpl/images/0706/3859/9462d.png)
![pOH =-log[OH^{-} ]= -log(2.3 \times 10^{-3} M) = 2.6](/tpl/images/0706/3859/e37b1.png)