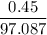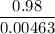Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3
You know the right answer?
If 0.98 g of an unknown was dissolved in 10.30 g of solvent and the resulting solution has a molalit...
Questions


Mathematics, 11.02.2022 23:50

Chemistry, 11.02.2022 23:50

History, 11.02.2022 23:50


History, 11.02.2022 23:50





Social Studies, 11.02.2022 23:50

Mathematics, 11.02.2022 23:50




World Languages, 12.02.2022 01:00

Mathematics, 12.02.2022 01:00

History, 12.02.2022 01:00

Social Studies, 12.02.2022 01:00

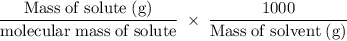 ......(i)
......(i)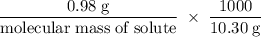
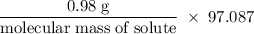
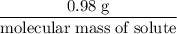 =
=