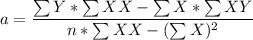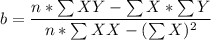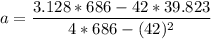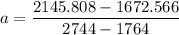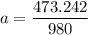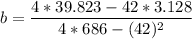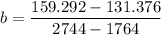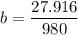Chemistry, 13.07.2020 23:01 Cheesygodxx
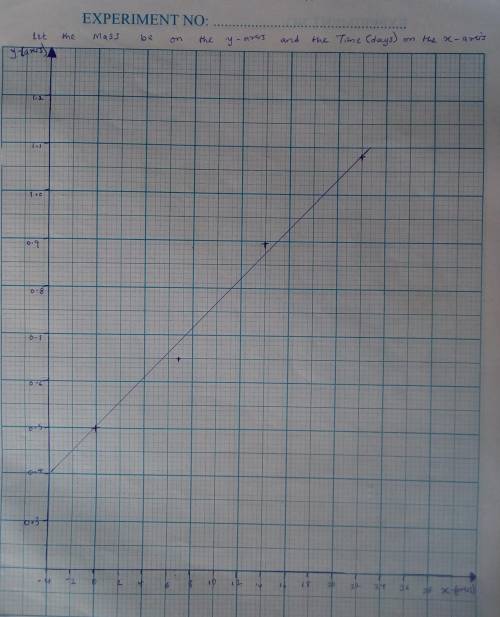
A seed of CuSO4.5H20 with a mass of 0.500 g was carefully placed into a saturated solution of copper (II) sulfate. After 7 days the mass of the seed crystal was determined to be 0.648 g. After 14 days the mass of the crystal increased to 0.899 g and after 21 days the mass of the crystal was found to be 1.081 g. Make a plot of mass vs time (days) and extrapolate to predict what would be the mass of the crystal in 28 days if the growth is linear. Include labels and units on each axis.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3

Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2

Chemistry, 23.06.2019 02:00
What is the source of continuous heat and energy that we receive from the sun
Answers: 2
You know the right answer?
A seed of CuSO4.5H20 with a mass of 0.500 g was carefully placed into a saturated solution of copper...
Questions


History, 06.10.2019 16:30


Mathematics, 06.10.2019 16:30

Biology, 06.10.2019 16:30



History, 06.10.2019 16:30


Mathematics, 06.10.2019 16:30





Mathematics, 06.10.2019 16:30


History, 06.10.2019 16:30

Mathematics, 06.10.2019 16:30


Biology, 06.10.2019 16:30

 :42 3.128 39.823 686
:42 3.128 39.823 686