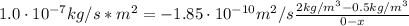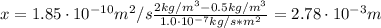A sheet of steel 2.5-mm thick has nitrogen atmospheres on both sides at 900"C and is permitted to achieve a steady-state diffusion condition. The diffusion coefficient for nitrogen in steel at this temperature is 1.85 x 10^-10 m^2/s, and the diffusion flux is found to be 1.0 x 10^-7 kg/m^2 s. Also, it is known that the concentration of nitrogen in the steel at the high-pressure surface is 2 kg/m^3. How far into the sheet from this high-pressure side will the concentration be 0.5 kg/m3? Assume a linear concentration profile.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2



Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1
You know the right answer?
A sheet of steel 2.5-mm thick has nitrogen atmospheres on both sides at 900"C and is permitted to ac...
Questions


Mathematics, 10.12.2020 07:50

Mathematics, 10.12.2020 07:50

Mathematics, 10.12.2020 07:50

Biology, 10.12.2020 07:50



English, 10.12.2020 07:50

History, 10.12.2020 07:50

Mathematics, 10.12.2020 07:50

Biology, 10.12.2020 07:50


Mathematics, 10.12.2020 07:50



Spanish, 10.12.2020 07:50


French, 10.12.2020 07:50

Mathematics, 10.12.2020 07:50

History, 10.12.2020 07:50

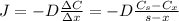
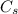 : is the nitrogen concentration in the surface of steel = 2 kg/m³
: is the nitrogen concentration in the surface of steel = 2 kg/m³ 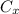 : is the nitrogen concentration in the point x = 0.5 kg/m³
: is the nitrogen concentration in the point x = 0.5 kg/m³