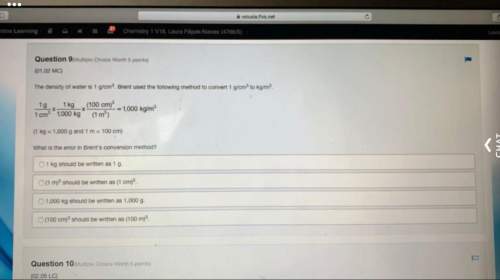
ons 9-12 OnOff Item 7 What can be calculated if the concentration of a reaction’s products and reactants are known, as well as the coefficients of each in the balanced equation? the rate of the reaction Gibbs free energy the entropy of the reaction the equilibrium constant

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

Chemistry, 23.06.2019 00:00
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1
You know the right answer?
ons 9-12 OnOff Item 7 What can be calculated if the concentration of a reaction’s products and react...
Questions

Mathematics, 03.02.2020 08:49


Biology, 03.02.2020 08:49

Mathematics, 03.02.2020 08:49




Mathematics, 03.02.2020 08:49

Chemistry, 03.02.2020 08:49


History, 03.02.2020 08:49

History, 03.02.2020 08:49




History, 03.02.2020 08:49




History, 03.02.2020 08:49