
Chemistry, 13.07.2020 19:01 jazminpagan15
The electron in a hydrogen atom, originally in level n = 8, undergoes a transition to a lower level by emitting a photon of wavelength 3745 nm. What is the final level of the electron?(c=3.00×10^8m/s, h=6.63×10^-34 J·s, RH=2.179×106-18J)a. 5
b. 6
c. 8
d. 9
e. 1

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2
You know the right answer?
The electron in a hydrogen atom, originally in level n = 8, undergoes a transition to a lower level...
Questions

Social Studies, 30.01.2020 02:43





Mathematics, 30.01.2020 02:43



English, 30.01.2020 02:43

Mathematics, 30.01.2020 02:43


Mathematics, 30.01.2020 02:43

Mathematics, 30.01.2020 02:43

Health, 30.01.2020 02:43



English, 30.01.2020 02:43

Mathematics, 30.01.2020 02:43


Mathematics, 30.01.2020 02:43

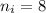
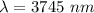
![\Delta E=R_H[\dfrac{1}{n_f^2}-\dfrac{1}{n_i^2}]](/tpl/images/0703/9206/6f427.png)
![\dfrac{hc}{\lambda}=R_H[\dfrac{1}{n_f^2}-\dfrac{1}{n_i^2}]](/tpl/images/0703/9206/68620.png)
![\dfrac{6.63\times 10^{-34}\times 3\times 10^8}{3745\times 10^{-9}}=2.179\times 10^{-18}[\dfrac{1}{n_f^2}-\dfrac{1}{8^2}]\\\\\dfrac{5.31\times 10^{-20}}{2.179\times 10^{-18}}=[\dfrac{1}{n_f^2}-\dfrac{1}{8^2}]\\\\0.0243=[\dfrac{1}{n_f^2}-\dfrac{1}{64}]\\\\0.0243+\dfrac{1}{64}=\dfrac{1}{n_f^2}\\\\0.039925=\dfrac{1}{n_f^2}\\\\n_f^2=25\\\\n_f=5](/tpl/images/0703/9206/7d6e7.png)


