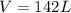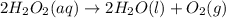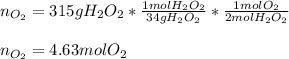Chemistry, 14.07.2020 17:01 frankcaroccio0203
9.Hydrogen peroxide decomposes to form water and oxygen gas according to the following equation:
2H2O2(aq) to 2H2O(l) + O2(g)
If 315 g of hydrogen peroxide, H2O2, decomposes and all the O2 gas is collected in a balloon at 0.792 atm and 23 degrees C, what is the volume of the O2 gas collected?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2


Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3
You know the right answer?
9.Hydrogen peroxide decomposes to form water and oxygen gas according to the following equation:
2H...
Questions

Computers and Technology, 02.03.2021 04:10

Mathematics, 02.03.2021 04:10


English, 02.03.2021 04:10

Law, 02.03.2021 04:10

Geography, 02.03.2021 04:10

Mathematics, 02.03.2021 04:10


Mathematics, 02.03.2021 04:10

History, 02.03.2021 04:10





Mathematics, 02.03.2021 04:10

History, 02.03.2021 04:10