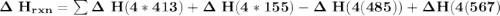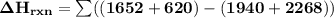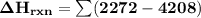Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1
You know the right answer?
Using bond energies, estimate the enthalpy of reaction for the following chemical reaction. CH4(g) +...
Questions

History, 22.04.2021 03:40

History, 22.04.2021 03:40

Mathematics, 22.04.2021 03:40

Social Studies, 22.04.2021 03:40

History, 22.04.2021 03:40



Chemistry, 22.04.2021 03:40

History, 22.04.2021 03:40

Mathematics, 22.04.2021 03:40

Mathematics, 22.04.2021 03:40

History, 22.04.2021 03:40



Mathematics, 22.04.2021 03:40

History, 22.04.2021 03:40



Biology, 22.04.2021 03:40

History, 22.04.2021 03:40

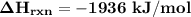
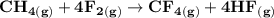
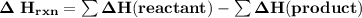
![\mathbf{\Delta \ H_{rxn} = \sum [\Delta H \ 4( C-H) + \Delta H \ 4(F-F) ]- \sum[ \Delta H \ 4( C-F)+\Delta H \ 4( H-F)] (product)}](/tpl/images/0703/4884/35173.png)