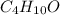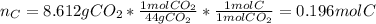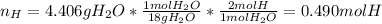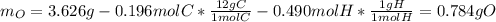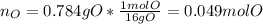Chemistry, 09.07.2020 02:01 germainenez3288
The compound known as diethyl ether, commonly referred to as ether, contains carbon, hydrogen, and oxygen. A 3.626 g sample of ether was combusted in an oxygen rich environment to produce 8.612 g of CO2(g) and 4.406 g of H2O(g). Insert subscripts to complete the empirical formula of ether.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
The compound known as diethyl ether, commonly referred to as ether, contains carbon, hydrogen, and o...
Questions

History, 23.12.2019 06:31


Social Studies, 23.12.2019 06:31

Geography, 23.12.2019 06:31



Social Studies, 23.12.2019 06:31




Social Studies, 23.12.2019 06:31

Physics, 23.12.2019 06:31

History, 23.12.2019 06:31


Geography, 23.12.2019 06:31

Mathematics, 23.12.2019 06:31

English, 23.12.2019 06:31

Mathematics, 23.12.2019 06:31