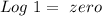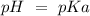Chemistry, 09.07.2020 01:01 jybuccaneers2022
50 mL of 0.1 M acetic acid is mixed with 50 mL of 0.1 M sodium acetate (the conjugate base). The Ka of acetic acid is approximately 1. 74 X 10 -5. What is the pH of the resulting solution?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Put these processes of the water cycle in the correct order, starting at the point where the water is in the lake: 1. water evaporates into the atmosphere 2. rain, snow, or other precipitation falls 3. water collects into larger bodies of water 4. water vapor condenses into liquid water
Answers: 1

Chemistry, 22.06.2019 00:10
Which of the following best describes the formation of plasma?
Answers: 1


You know the right answer?
50 mL of 0.1 M acetic acid is mixed with 50 mL of 0.1 M sodium acetate (the conjugate base). The Ka...
Questions

History, 19.11.2019 01:31


Mathematics, 19.11.2019 01:31

Mathematics, 19.11.2019 01:31

Geography, 19.11.2019 01:31


Biology, 19.11.2019 01:31

Mathematics, 19.11.2019 01:31




English, 19.11.2019 01:31




History, 19.11.2019 01:31


Mathematics, 19.11.2019 01:31


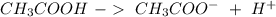
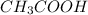 ) and a base (
) and a base (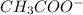 ). Therefore we can write the henderson-hasselbach reaction:
). Therefore we can write the henderson-hasselbach reaction:![pH~=~pKa+Log\frac{[CH_3COO^-]}{[CH_3COOH]}](/tpl/images/0703/3490/99062.png)
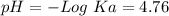
![[CH_3COO^-]=[CH_3COOH]](/tpl/images/0703/3490/ee54c.png)
![\frac{[CH_3COO^-]}{[CH_3COOH]}~=~1](/tpl/images/0703/3490/6e489.png)