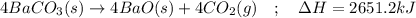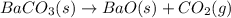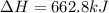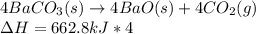Given the thermochemical expression
BaO (s) + CO2 (g) =
BaCO3(s)
AH° = -662.8 kJ
...

Chemistry, 09.07.2020 01:01 Paytonsmommy09
Given the thermochemical expression
BaO (s) + CO2 (g) =
BaCO3(s)
AH° = -662.8 kJ
Write the thermochemical expression for the production of 4 mol CO2 by decomposition of solid
barium carbonate.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2



Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
Questions



Mathematics, 18.07.2019 03:00



Biology, 18.07.2019 03:00

Social Studies, 18.07.2019 03:00


Business, 18.07.2019 03:00



English, 18.07.2019 03:00


Mathematics, 18.07.2019 03:00


Biology, 18.07.2019 03:00

Mathematics, 18.07.2019 03:00

Mathematics, 18.07.2019 03:00