Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 23:00
The wave shown on the electromagnetic spectrum disturb the medium it passes through a)different frequency. b)the same frequency .
Answers: 2

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2
You know the right answer?
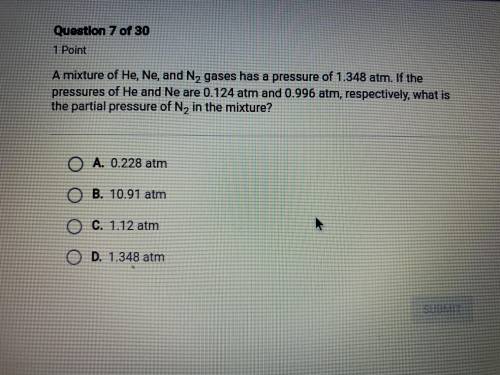
a mixture of He, Ne, and N2 gases has a pressure of 1.348 atm. if the pressures of He and Ne are 0.1...
Questions




Mathematics, 20.02.2020 04:31





Social Studies, 20.02.2020 04:31








Computers and Technology, 20.02.2020 04:31


Mathematics, 20.02.2020 04:31