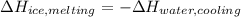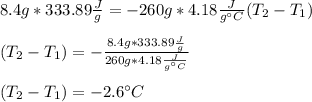An 8.4 g ice cube is placed into 260 g of water. Calculate the temperature change in the water upon the complete melting of the ice. Assume that all of the energy required to melt the ice comes from the water. Express your answer in terms of the initial temperature of water, T. a. -0.033T - 2.6 °Cb. -2.6T + 0.033 °Cc. 0.033T - 2.6 °Cd. 2.6T -0.033 °C

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1
You know the right answer?
An 8.4 g ice cube is placed into 260 g of water. Calculate the temperature change in the water upon...
Questions



Mathematics, 05.05.2020 03:20

Mathematics, 05.05.2020 03:20

Mathematics, 05.05.2020 03:20




Mathematics, 05.05.2020 03:20




History, 05.05.2020 03:20






Social Studies, 05.05.2020 03:20