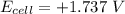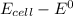Chemistry, 08.07.2020 02:01 gabbytumey
Consider an electrochemical cell based on the spontaneous reaction 2AgCl(s) + Zn(s) → 2Ag(s) + 2Cl– + Zn2+. If the zinc ion concentration is kept constant at 1 M, and the chlorine ion concentration is decreased from 1 M to 0.001 M, the cell voltage should:

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

You know the right answer?
Consider an electrochemical cell based on the spontaneous reaction 2AgCl(s) + Zn(s) → 2Ag(s) + 2Cl–...
Questions


Biology, 24.11.2021 14:20



Social Studies, 24.11.2021 14:20


Advanced Placement (AP), 24.11.2021 14:20

Computers and Technology, 24.11.2021 14:20

History, 24.11.2021 14:20



Physics, 24.11.2021 14:20


Physics, 24.11.2021 14:20

Mathematics, 24.11.2021 14:20

Social Studies, 24.11.2021 14:20

Advanced Placement (AP), 24.11.2021 14:20



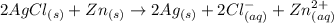
![E_{cell} = E^0- \dfrac{0,059}{n}log (\dfrac{[product]}{[reactant]})](/tpl/images/0702/8732/cb883.png)
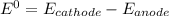
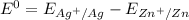
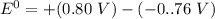
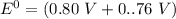
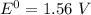
![E_{cell} = E^0- \dfrac{0.059}{n}log (\dfrac{[product]}{[reactant]})](/tpl/images/0702/8732/6f93c.png)
![E_{cell} = 1.56 - \dfrac{0.059}{2}log ({[Zn^{2+} ]}{[Cl^{2-}]})](/tpl/images/0702/8732/feb0e.png)
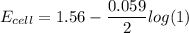
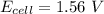
![E_{cell} = 1.56 - \dfrac{0.059}{2}log ({[1*0.001^2}]})](/tpl/images/0702/8732/27a68.png)
![E_{cell} = 1.56 - 0.0295 \ * \ log ({[1*10^{-6}}]})](/tpl/images/0702/8732/5785a.png)