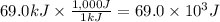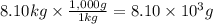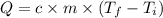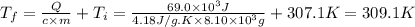Chemistry, 08.07.2020 02:01 sanchezp0821
A chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 8.10kg of water at 33.9 degrees celsius . During the reaction 69.0kJ of heat flows out of the bath and into the flask. Calculate the new temperature of the water bath. You can assume the specific heat capacity of water under these conditions is 4.18J*g*K.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

Chemistry, 23.06.2019 00:10
Find the missing probability in the table below a.0.10 b.40 c.0.80 d. 0.20
Answers: 2

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
You know the right answer?
A chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 8....
Questions





Mathematics, 24.05.2020 06:57


Health, 24.05.2020 06:57

History, 24.05.2020 06:57

Chemistry, 24.05.2020 06:57

Mathematics, 24.05.2020 06:57

Mathematics, 24.05.2020 06:57

Mathematics, 24.05.2020 06:57



Biology, 24.05.2020 06:57




Mathematics, 24.05.2020 06:57

Mathematics, 24.05.2020 06:57