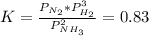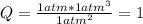Chemistry, 07.07.2020 21:01 kingtrent81
For the reaction below, initially the partial pressure of all 3 gases is 1.0atm. . 2NH3(g)--> N2(g) + 3H2(g) K, 0.83 1. When the reaction reach equilibrium the partial pressure of N2 will be greater than 1atm The reaction would shift toward the reactants The reaction would shift toward the products 2. When the reaction reach equilibrium the partial pressure of NH3 will be greater than 1atm 3. When the reaction reach equilibrium the partial pressure of H2 will be greater than 1atm

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2
You know the right answer?
For the reaction below, initially the partial pressure of all 3 gases is 1.0atm. . 2NH3(g)--> N2(...
Questions

English, 02.10.2020 14:01





Mathematics, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01



Computers and Technology, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01



Mathematics, 02.10.2020 14:01

Biology, 02.10.2020 14:01

History, 02.10.2020 14:01

Biology, 02.10.2020 14:01

English, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01