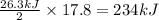Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1


Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 23.06.2019 04:10
In an experiment, 45g of silicon tetrachloride are treated with 45ml of water. what is the theoretical yield in grams of hcl
Answers: 3
You know the right answer?
The reduction of iron(III) oxide to iron metal is an endothermic process: Fe2O3(s) + 2 CO(g) → 2 Fe(...
Questions


Mathematics, 25.01.2021 22:20

Mathematics, 25.01.2021 22:20


Social Studies, 25.01.2021 22:20

English, 25.01.2021 22:20


History, 25.01.2021 22:20


English, 25.01.2021 22:20



Chemistry, 25.01.2021 22:20

Mathematics, 25.01.2021 22:20

Mathematics, 25.01.2021 22:20




Mathematics, 25.01.2021 22:20

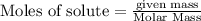
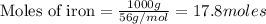 (1.00kg=1000g)
(1.00kg=1000g)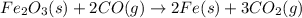
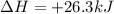
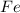 is produced = 26.3 kJ
is produced = 26.3 kJ