Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Asample of silver (with work function ? = 4.52 ev) is exposed to an ultraviolet light source (? = 200 nm), which results in the ejection of photoelectrons. what changes will be observed if: silver is replaced with copper (? = 5.10 ev) more photoelectrons ejected no photoelectrons are emitted fewer photoelectrons ejected more energetic photoelectrons (on average) less energetic photoelectrons (on average)
Answers: 3

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3
You know the right answer?
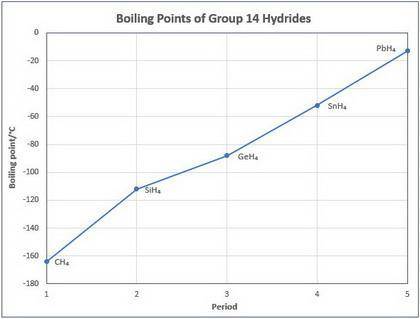
1) Los compuestos binarios de hidrogeno de los elementos del grupo 4A y sus puntos de ebullición son...
Questions

Mathematics, 28.01.2021 01:00



Mathematics, 28.01.2021 01:00



Mathematics, 28.01.2021 01:00

Spanish, 28.01.2021 01:00



Mathematics, 28.01.2021 01:00

Spanish, 28.01.2021 01:00


English, 28.01.2021 01:00



History, 28.01.2021 01:00



Mathematics, 28.01.2021 01:00