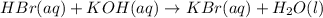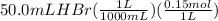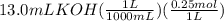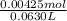Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1
You know the right answer?
A50.0ml- volume of 0.15m hbr is titrated with 0.25m koh . calculate the ph after the addition of 13....
Questions

Mathematics, 01.07.2019 13:30

Arts, 01.07.2019 13:30

Social Studies, 01.07.2019 13:30

Biology, 01.07.2019 13:30

Social Studies, 01.07.2019 13:30

Social Studies, 01.07.2019 13:30

History, 01.07.2019 13:30



Mathematics, 01.07.2019 13:30

Mathematics, 01.07.2019 13:30





Mathematics, 01.07.2019 13:30

Health, 01.07.2019 13:30



History, 01.07.2019 13:30