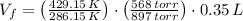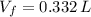Chemistry, 05.07.2020 14:01 gameranonymous266
A sample of 0.35 L of argon gas (at a temperature of 13 oC and a pressure of 568 torr) is heated to 156 oC and a new pressure of 897 torr. Calculate the new volume of the gas.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1
You know the right answer?
A sample of 0.35 L of argon gas (at a temperature of 13 oC and a pressure of 568 torr) is heated to...
Questions

Mathematics, 27.01.2021 07:00

Biology, 27.01.2021 07:00

Mathematics, 27.01.2021 07:00


Mathematics, 27.01.2021 07:00


Mathematics, 27.01.2021 07:00


Mathematics, 27.01.2021 07:00


Mathematics, 27.01.2021 07:00

Mathematics, 27.01.2021 07:00


Mathematics, 27.01.2021 07:00

Mathematics, 27.01.2021 07:00

English, 27.01.2021 07:00



Spanish, 27.01.2021 07:00

History, 27.01.2021 07:00

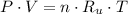
 - Pressure, measured in torr.
- Pressure, measured in torr.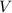 - Volume, measured in liters.
- Volume, measured in liters.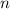 - Molar quantity, measured in moles.
- Molar quantity, measured in moles. 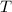 - Temperature, measured in kelvins.
- Temperature, measured in kelvins.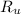 - Ideal gas constant, measured in
- Ideal gas constant, measured in 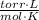 .
.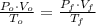
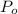 ,
, 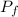 - Initial and final pressures, measured in torr.
- Initial and final pressures, measured in torr.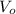 ,
, 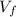 - Initial and final volumes, measured in liters.
- Initial and final volumes, measured in liters.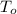 ,
, 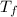 - Initial and final temperatures, measured in kelvins.
- Initial and final temperatures, measured in kelvins. 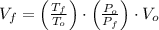
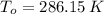 ,
, 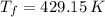 ,
, 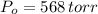 ,
, 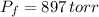 and
and 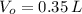 , the new volume of the gas is:
, the new volume of the gas is: