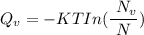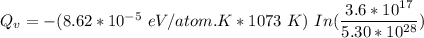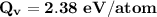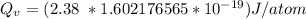Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Measuring which physical property is most likely to produce the most precise results when trying to identify a substance
Answers: 1

Chemistry, 21.06.2019 16:20
What would you do if you told the guy you liked that you liked him
Answers: 1

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

You know the right answer?
Calculate the energy (in J/atom) for vacancy formation in silver, given that the equilibrium number...
Questions


English, 18.12.2020 19:10


English, 18.12.2020 19:10

Mathematics, 18.12.2020 19:10

Mathematics, 18.12.2020 19:10

Mathematics, 18.12.2020 19:10

Chemistry, 18.12.2020 19:10


History, 18.12.2020 19:10


History, 18.12.2020 19:10



Computers and Technology, 18.12.2020 19:10

Mathematics, 18.12.2020 19:10




Mathematics, 18.12.2020 19:10

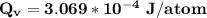
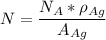
 avogadro's number =
avogadro's number = 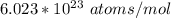
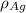 = Density of silver = 9.5 g/cm³
= Density of silver = 9.5 g/cm³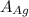 = Atomic weight of sliver = 107.9 g/mol
= Atomic weight of sliver = 107.9 g/mol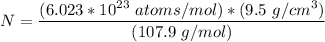
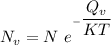
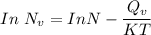
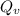 the subject of the formula; we have:
the subject of the formula; we have: