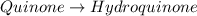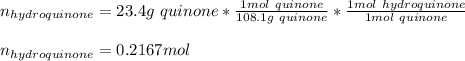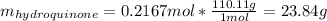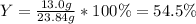Chemistry, 04.07.2020 01:01 allieb12334
Determine the theoretical maximum moles of hydroquinone, , that could be produced in this experiment. The reactant, quinone, is the limiting reagent. (To avoid introducing rounding errors on intermediate calculations, enter your answer to four significant figures.)
Reactant mass 23.4g
Product mass 13.0g
Reactant moles 0.2167 mol
Reactant mass 23.4g
Product mass 13.0g
Molar mass C 12.0 g/mol
Molar mass H 1.00 g/mol
Molar mass O 16.0 g/mol
Theoretical maximum moles of hydroquinone:

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2
You know the right answer?
Determine the theoretical maximum moles of hydroquinone, , that could be produced in this experiment...
Questions



Mathematics, 22.04.2021 21:20




English, 22.04.2021 21:20

Mathematics, 22.04.2021 21:20

Arts, 22.04.2021 21:20

Social Studies, 22.04.2021 21:20


History, 22.04.2021 21:20


Social Studies, 22.04.2021 21:20

Mathematics, 22.04.2021 21:20

Mathematics, 22.04.2021 21:20


History, 22.04.2021 21:20


Advanced Placement (AP), 22.04.2021 21:20