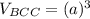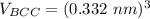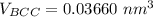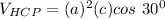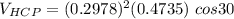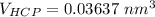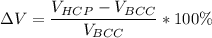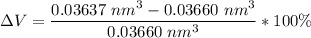Chemistry, 03.07.2020 23:01 harmonytaylor13
Above 882oC, zirconium has a BCC crystal structure with a = 0.332 nm. Below this temperature, zirconium has an HCP structure with a = 0.2978 nm and c = 0.4735 nm. Determine the percent volume change when BCC zirconium transforms to HCP zirconium. Is that contraction or expansion?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 23.06.2019 03:30
Name atleast 3 type of energy associated with the microwave
Answers: 1

Chemistry, 23.06.2019 07:00
Ajar contains a certain substance. which observation would show that the substance must be either a solid or a liquid?
Answers: 1
You know the right answer?
Above 882oC, zirconium has a BCC crystal structure with a = 0.332 nm. Below this temperature, zircon...
Questions




Mathematics, 22.01.2021 19:40








Social Studies, 22.01.2021 19:40

Mathematics, 22.01.2021 19:40

Chemistry, 22.01.2021 19:40

Mathematics, 22.01.2021 19:40



Mathematics, 22.01.2021 19:40

Mathematics, 22.01.2021 19:40

Mathematics, 22.01.2021 19:40