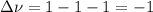Chemistry, 03.07.2020 23:01 austinbeesley9855
Consider the following system at equilibrium where delta H° = -108 kJ, and Kc = 77.5, at 600 K. CO(g) + Cl2(g) <---> COCl2(g) If the VOLUME of the equilibrium system is suddenly decreased at constant temperature:

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 00:10
Find the missing probability in the table below a.0.10 b.40 c.0.80 d. 0.20
Answers: 2


Chemistry, 23.06.2019 04:00
Which method would be best to separate a mixture of sand and gravel
Answers: 1

Chemistry, 23.06.2019 04:00
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2
You know the right answer?
Consider the following system at equilibrium where delta H° = -108 kJ, and Kc = 77.5, at 600 K. CO(g...
Questions



Mathematics, 20.11.2020 01:20


History, 20.11.2020 01:20


Advanced Placement (AP), 20.11.2020 01:20


Chemistry, 20.11.2020 01:20

English, 20.11.2020 01:20




Mathematics, 20.11.2020 01:20

History, 20.11.2020 01:20

History, 20.11.2020 01:20




Mathematics, 20.11.2020 01:20