Chemistry, 03.07.2020 20:01 kwoolfe59006
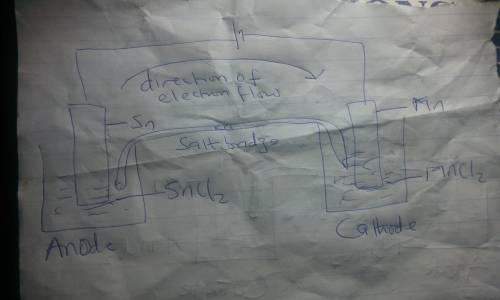
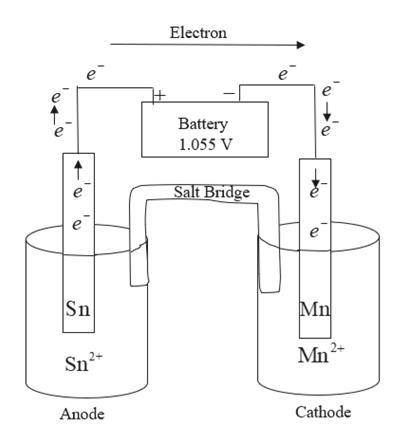
Draw an electrolytic cell in which Mn2+ is reduced to Mn and Sn is oxidized to Sn2+. (Assume standard conditions).
Part A Label the anode and cathode, indicate the direction of electron flow.
Drag the appropriate labels to their respective targets.
Part B Write an equation for the half-reaction occurring at each electrode.
Express your answers as chemical equations separated by a comma. Identify all of the phases in your answer.
Part C What minimum voltage is necessary to drive the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2


Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1
You know the right answer?
Draw an electrolytic cell in which Mn2+ is reduced to Mn and Sn is oxidized to Sn2+. (Assume standar...
Questions




History, 14.12.2020 20:40


Mathematics, 14.12.2020 20:40


Mathematics, 14.12.2020 20:40


Mathematics, 14.12.2020 20:40



Biology, 14.12.2020 20:40

Mathematics, 14.12.2020 20:40


Physics, 14.12.2020 20:40

Chemistry, 14.12.2020 20:40