

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3

Chemistry, 23.06.2019 04:20
The lewis diagrams for magnesium and fluorine are shown below. what is the correct chemical formula for magnesium fluoride? a. mgf b. mg2f c. mgf2 d. mg2f3
Answers: 1
You know the right answer?
Consider the following intermediate chemical equations.(IMAGE) -205.7 kJ -113.4 kJ -14.3 kJ 78.0 kJ...
Questions



Health, 15.12.2019 11:31





Biology, 15.12.2019 11:31

Mathematics, 15.12.2019 11:31



Mathematics, 15.12.2019 11:31



Mathematics, 15.12.2019 11:31

Biology, 15.12.2019 11:31

Chemistry, 15.12.2019 11:31



Mathematics, 15.12.2019 11:31

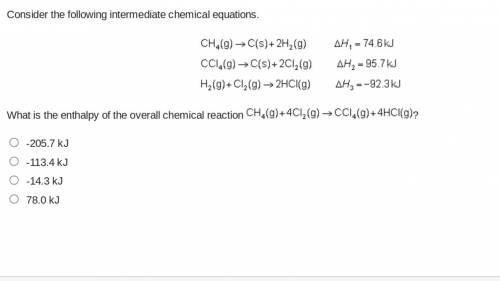
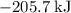 .
.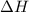 values) be combined to produce the reaction
values) be combined to produce the reaction 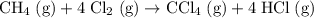 ?
?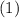 ,
, 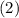 , and
, and 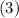 denote the three reactions with know
denote the three reactions with know 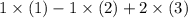 will give the required reaction
will give the required reaction 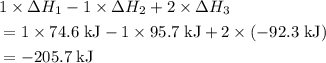 .
. 


