
Chemistry, 02.07.2020 09:01 luvpeaceandsocc3678
Consider the chemical reaction below. Zn(s) + 2H+(aq) -> Zn2+(aq) + H2(g). Which half reaction correctly represents reduction for this equation?
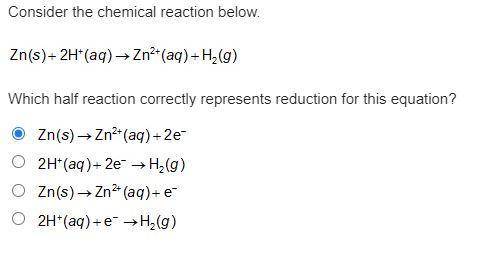

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
Consider the chemical reaction below. Zn(s) + 2H+(aq) -> Zn2+(aq) + H2(g). Which half reaction co...
Questions

Computers and Technology, 18.02.2022 02:40

Biology, 18.02.2022 02:40




Mathematics, 18.02.2022 02:40







Mathematics, 18.02.2022 02:50



Mathematics, 18.02.2022 02:50

Mathematics, 18.02.2022 02:50





