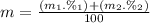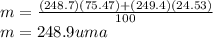Chemistry, 02.10.2019 14:50 Jsquad8879
Calculate the average atomic mas of an unknown element that has two naturally-occurring isotopes.
the first isotope occurs 75.47% of the time and has a mass of 248.7 a. m.u.
the second isotope occurs 24.53% of the time and has a mass of 249.4 a. m.u.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
How much heat is released upon converting one mole of steam (18.0 g) from 100.0 ∘c to water at 25.0 ∘c? show work and constants, trying to figure out how it works. only given the heat capacity for steam and water so try to only use that
Answers: 1

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1
You know the right answer?
Calculate the average atomic mas of an unknown element that has two naturally-occurring isotopes.
Questions

Social Studies, 03.08.2019 01:30


Biology, 03.08.2019 01:30


Computers and Technology, 03.08.2019 01:30




Health, 03.08.2019 01:30


Mathematics, 03.08.2019 01:30

Biology, 03.08.2019 01:30



Geography, 03.08.2019 01:30

History, 03.08.2019 01:30



Spanish, 03.08.2019 01:30