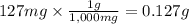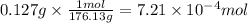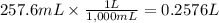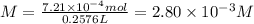Chemistry, 01.07.2020 16:01 Roninsongrant
Given that one cup = 257.6 mL, calculate the molarity of vitamin C in orange juice. Express your answer to an appropriate number of significant figures with the appropriate units.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1
You know the right answer?
Given that one cup = 257.6 mL, calculate the molarity of vitamin C in orange juice.
Express your an...
Questions

Mathematics, 21.11.2020 04:20


Mathematics, 21.11.2020 04:20

Mathematics, 21.11.2020 04:20

Mathematics, 21.11.2020 04:20

Mathematics, 21.11.2020 04:20

History, 21.11.2020 04:20

Social Studies, 21.11.2020 04:20



Mathematics, 21.11.2020 04:20

Mathematics, 21.11.2020 04:20

Mathematics, 21.11.2020 04:20


World Languages, 21.11.2020 04:30


Biology, 21.11.2020 04:30



Social Studies, 21.11.2020 04:30