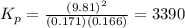Chemistry, 29.06.2020 20:01 lflugo6oyn4sp
At a particular temperature, an equilibrium mixture the reaction below was found to contain 0.171 atm of I2, 0.166 atm of Cl2 and 9.81 atm of ICl. Calculate the value of the equilibrium constant, Kp at this temperature. I2(g) + Cl2(g) <=> 2 ICl(g)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2

Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1
You know the right answer?
At a particular temperature, an equilibrium mixture the reaction below was found to contain 0.171 at...
Questions

Business, 21.12.2020 20:50


Mathematics, 21.12.2020 20:50

Mathematics, 21.12.2020 20:50

English, 21.12.2020 20:50





Social Studies, 21.12.2020 20:50

History, 21.12.2020 20:50

Mathematics, 21.12.2020 20:50



English, 21.12.2020 20:50

Mathematics, 21.12.2020 20:50


Chemistry, 21.12.2020 20:50

Social Studies, 21.12.2020 20:50

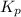 .
.![K_{p} =\frac{[ICl]^2}{[I_{2}][Cl_{2}] }](/tpl/images/0696/9637/8ab45.png)