Chemistry, 29.06.2020 20:01 mdaniella522
At a given temperature, 3.12 atm of H2 and 5.52 atm of I2 are mixed and allowed to come to equilibrium. The equilibrium pressure of HI is found to be 1.738 atm. Calculate Kp for the reaction at this temperature. H2(g) + I2(g) <=> 2 HI(g). Give your answer to 3 decimal places.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 07:20
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1
You know the right answer?
At a given temperature, 3.12 atm of H2 and 5.52 atm of I2 are mixed and allowed to come to equilibri...
Questions

Mathematics, 22.09.2021 08:40


Mathematics, 22.09.2021 08:40


Mathematics, 22.09.2021 08:40





Physics, 22.09.2021 08:40

Social Studies, 22.09.2021 08:40

English, 22.09.2021 08:40

Engineering, 22.09.2021 08:40

Mathematics, 22.09.2021 08:40


Mathematics, 22.09.2021 08:40

Mathematics, 22.09.2021 08:40



Mathematics, 22.09.2021 08:40

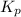 . To do so, we will need to use the ICE chart. The I in ICE is initial quantity. In this case, it is the initial pressure. Pressure is in atm. The C in ICE is change in each quantity. The E is equilibrium.
. To do so, we will need to use the ICE chart. The I in ICE is initial quantity. In this case, it is the initial pressure. Pressure is in atm. The C in ICE is change in each quantity. The E is equilibrium.![K_{p} =\frac{[HI]^2}{[H_{2}][I_{2} ] }](/tpl/images/0696/9799/d09a4.png)