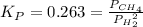C(s) + 2 H2(g) = CH,(9)
has Kp = 0.263 at 1000. K. Calculate the total pressure at equilibrium when 5.207 g of H, and 23.29 g of C(s) are placed in
a 9.32 L flask and heated to 1000, K.
atm
Protal =
Calculate the total pressure when 5.207 g of H, and 8.333 g of C(s) are placed in a 9.32 L flask and heated to 1000. K.
atm
Ptotal =

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
If a reaction has g of -136kj at 110°c, will it be spontaneous at this temperature (110°c)? yes or no
Answers: 2

Chemistry, 21.06.2019 21:30
Sex cells from female reproductive organ? 1) mitosis 2) fertilization 3) zygote 4) eggs 5) meiosis 6) sperm
Answers: 2

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2
You know the right answer?
C(s) + 2 H2(g) = CH,(9)
has Kp = 0.263 at 1000. K. Calculate the total pressure at equilibrium when...
Questions

Chemistry, 05.07.2019 00:30

Mathematics, 05.07.2019 00:30

Biology, 05.07.2019 00:30

Biology, 05.07.2019 00:30



Mathematics, 05.07.2019 00:30

Mathematics, 05.07.2019 00:30

Mathematics, 05.07.2019 00:30

Mathematics, 05.07.2019 00:30

Mathematics, 05.07.2019 00:30

Biology, 05.07.2019 00:30



Biology, 05.07.2019 00:30


Mathematics, 05.07.2019 00:30



Physics, 05.07.2019 00:30