
Chemistry, 29.06.2020 04:01 shawntawright1
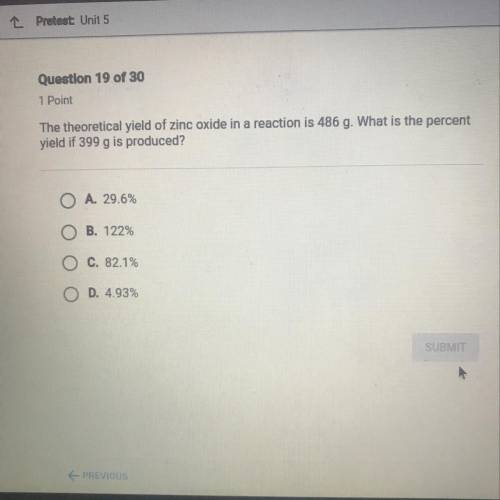
The theoretical yield of zinc oxide in a reaction is 486 g. What is the percent yield if 399 g is produced?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1
You know the right answer?
The theoretical yield of zinc oxide in a reaction is 486 g. What is the percent
yield if 399 g is p...
Questions


Mathematics, 09.03.2020 01:59




Biology, 09.03.2020 01:59



Mathematics, 09.03.2020 01:59


Social Studies, 09.03.2020 01:59


English, 09.03.2020 02:00

Advanced Placement (AP), 09.03.2020 02:00

Mathematics, 09.03.2020 02:00

English, 09.03.2020 02:00

Computers and Technology, 09.03.2020 02:01


English, 09.03.2020 02:01



