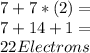Chemistry, 28.06.2020 07:01 tahiratnoel20
Give the number of lone pairs around the central atom and the geometry of the ion IBr2.
A. 3 lone pairs, linear
B. 2 lone pairs, bent
C. 0 lone pairs, linear
D. 1 lone pair, bent
E. 3 lone pairs, bent

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2
You know the right answer?
Give the number of lone pairs around the central atom and the geometry of the ion IBr2.
A. 3 lone p...
Questions

Mathematics, 05.10.2019 05:00

History, 05.10.2019 05:00



Business, 05.10.2019 05:00

Mathematics, 05.10.2019 05:00

History, 05.10.2019 05:00

Mathematics, 05.10.2019 05:00

Mathematics, 05.10.2019 05:00



Mathematics, 05.10.2019 05:00

Social Studies, 05.10.2019 05:00

Physics, 05.10.2019 05:00

Social Studies, 05.10.2019 05:00


Mathematics, 05.10.2019 05:00

History, 05.10.2019 05:00