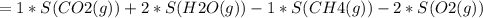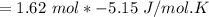Chemistry, 27.06.2020 18:01 destiny3200
Consider the reaction CH4(g) 2O2(g)CO2(g) 2H2O(g) Using standard thermodynamic data at 298K, calculate the entropy change for the surroundings when 1.62 moles of CH4(g) react at standard conditions.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

You know the right answer?
Consider the reaction CH4(g) 2O2(g)CO2(g) 2H2O(g) Using standard thermodynamic data at 298K, calcula...
Questions


Mathematics, 14.10.2019 11:30



Mathematics, 14.10.2019 11:30



Social Studies, 14.10.2019 11:30

Physics, 14.10.2019 11:30



Computers and Technology, 14.10.2019 11:30




Mathematics, 14.10.2019 11:30

Biology, 14.10.2019 11:30


Mathematics, 14.10.2019 11:30

Business, 14.10.2019 11:30