
The same reaction, with exactly the same amount of reactant, is conducted in a bomb calorimeter and in a coffee-cup calorimeter. In one measurement, qrxn=−13.3qrxn=−13.3 kJkJ in the other qrxn=−11.9qrxn=−11.9 kJkJ. Which value was obtained in the bomb calorimeter? (Assume that the reaction has a positive ΔVΔV in the coffee-cup calorimeter.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3
You know the right answer?
The same reaction, with exactly the same amount of reactant, is conducted in a bomb calorimeter and...
Questions




Mathematics, 20.04.2021 02:40


English, 20.04.2021 02:40


English, 20.04.2021 02:40

Mathematics, 20.04.2021 02:40


English, 20.04.2021 02:40

English, 20.04.2021 02:40

Mathematics, 20.04.2021 02:40




World Languages, 20.04.2021 02:40

Biology, 20.04.2021 02:40

Arts, 20.04.2021 02:40

Mathematics, 20.04.2021 02:40

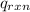 = -13.3 KJ belongs to the bomb calorimeter
= -13.3 KJ belongs to the bomb calorimeter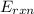 of the reaction, the work of the reaction
of the reaction, the work of the reaction 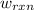 and the heat of the reaction
and the heat of the reaction 

